GMP Authorized User Roles and Permissions¶
User Roles and Permissions:¶
Role |
Responsibilities |
Permissions |
Restricted Actions |
|---|---|---|---|
Operator |
Conducting AUC experiments in accordance with standard operating procedures (SOPs), and ensuring raw data is properly collected |
Setting up and conducting experiments |
Modifying data after submission and approving or rejecting experiments. |
Reviewer |
Reviews experiment results for scientific accuracy and SOP compliance |
Access completed experiment data, and view audit trails. |
Conducting experiments, modifying data, and approving or rejecting experiments. |
Approver |
Verifies that the experiment conducted is compliant with SOPs, and that other compliance documentation is in order. |
Access completed experiment data, and view audit trails. |
Conducting experiments, modifying data, and validating scientific accuracy of experiments. |
Subject Matter Expert (SME) |
Provides expertise on scientific accuracy and experiment validity. |
Access completed experiment data. |
Conducting experiments, and modifying data. |
Role Assignment: Roles are assigned from the lab-specific UltraScan3 LIMS portal by a system administrator (Level 0 user), whose role is restricted to assigning user levels and roles. Each role is mutually exclusive to ensure a clear separation of responsibilities and maintain GMP compliance.
Note
The GMP module can be accessed using the drop-down ‘GMP’ menu located at the top of the UltraScan III module
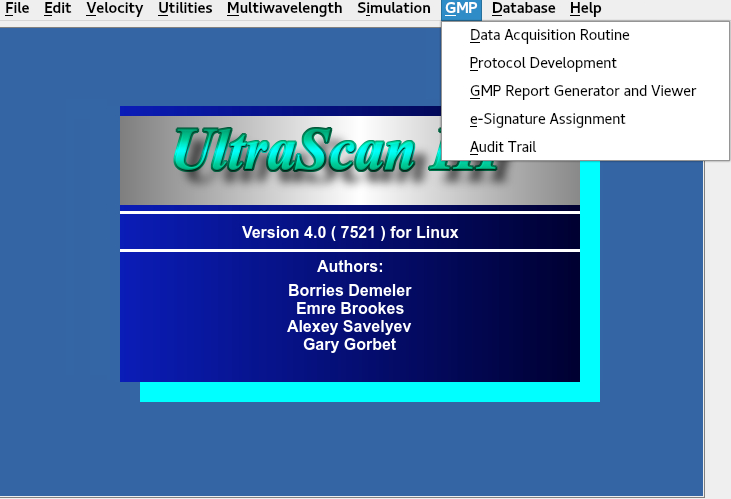
GMP Modules Access